With the success of the vaccine insight, it is important to understand that none of it would have been possible without years of expertise and funding support
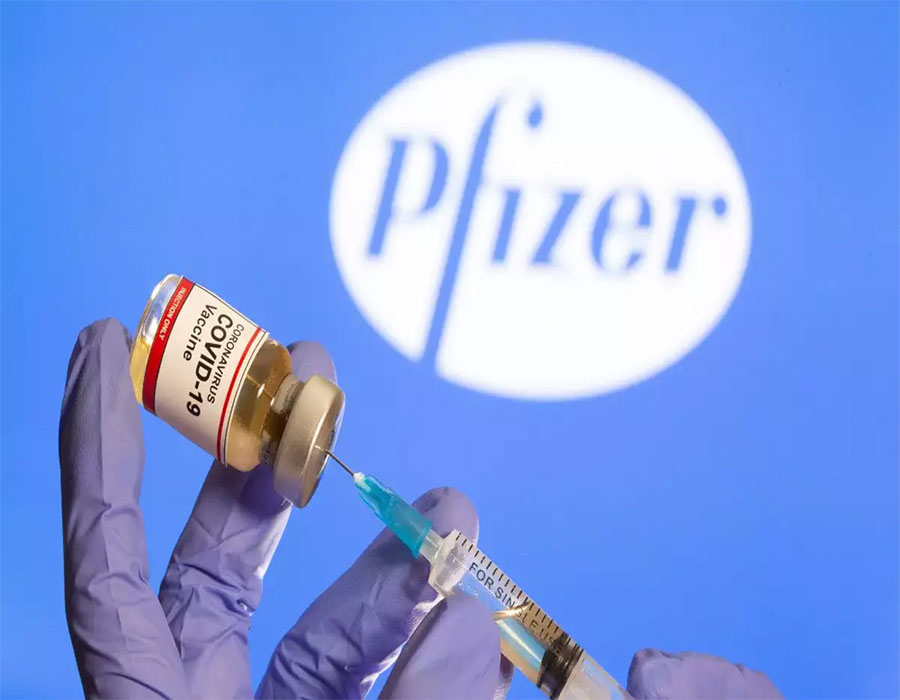
One of the most difficult years in recent memory, 2020 has been nothing less than a global healthcare nightmare. However, as the year draws to an end, there is good news on the horizon. Even as an indigenously-developed vaccine by Bharat Biotech has entered Phase-3 of clinical trials, several other international vaccines have either shown promising results or are on their way to reach last-stage trials. In the UK, the Medicines and Healthcare products Regulatory Agency (MHRA) has already given its nod to the Pfizer/BioNTech vaccine and the country is gearing up to start mass vaccination of its population. Meanwhile, the successful trials of the Oxford-AstraZeneca and Moderna vaccines have raised tremendous hopes too. While Moderna and Pfizer have announced high efficacy in clinical trials for their respective vaccines, that is up to 95 per cent, the Oxford-AstraZeneca vaccine can be stored in normal refrigerators. This makes it much easier and cheaper to store, transport and distribute in comparison to the other two candidates which would need to be stored at minus 20-80 °C.
Being cost-effective and easy to store, the Oxford vaccine is being touted as the most promising candidate for a country like India, which has cold chain issues. Russia’s Sputnik V, another such vaccine that stays stable between 2 and 8 °C, is also being considered viable for India and other developing countries.
These vaccines not only mark a defining moment for medical advances in the modern era, they are a manifestation of the commitment shown by scientists and researchers across the world in producing the shots in record time. They remind us how investing in medical research is critical to beating unforeseen health emergencies and vital to the survival of the human race.
Race against time: The stories behind the development of these vaccines and the speed at which they were done is truly inspiring. Let’s take the case of the Oxford vaccine. British and Irish scientists Professor Adrian Hill, Professor Sarah Gilbert and Professor Terese Lambe started working on this vaccine in early January as soon as scientists in China published the first genetic sequence of the virus. Given the novel nature of the Coronavirus, it was an uphill task but Oxford University and later the Government gave the vaccinologists access to unprecedented funding and resources to find the much-coveted COVID vaccine. The scientists based their work on an existing technology they pioneered and patented years ago in the Oxford labs. In fact, they designed this vaccine that very weekend itself in January, staying up all night. At that point it wasn’t even clear how fast the virus would spread or how many people it would end up infecting, but these researchers saw it as an opportunity to demonstrate rapid vaccine development against a new viral threat. From here on, within weeks Oxford had useable vaccines for lab tests and clinical grade shots for human trials. The rest, as they say is history.
By the beginning of April, the COVID19 virus had spread to most parts of the world with the UN chief Antonio Guterres terming it the “worst crisis” since World War II. With deaths mounting and economies crashing one after the other, the development of this vaccine was much more than a healthcare need, it was an economic urgency as well. The Oxford vaccine is a chimpanzee adenovirus-vectored shot. It uses a genetically-modified version of a virus strain that is usually found in chimpanzees. This modified virus carried with it an element of the Covid-19 viral strain that triggers an immune response in the human body.
Not without controversies: Given the urgency and the competition between pharma companies, vaccine development has understandably been hasty. The Oxford vaccine trial discovered up to 90 per cent efficacy when the volunteers were given a half dose before a full dose booster shot. Interestingly, when given two full doses, the vaccine showed only 62 per cent efficacy. While this serendipitous discovery gave an expected boost to the vaccine, questions remain over the cause of this surprising trial result. It also brought to the fore discrepancy in the narratives of the two partnering agencies on the project. While the AstraZeneca team insisted that half dosage was given as an inadvertent error, Oxford scientists underlined that it was a calibrated move.
Even as the varying accounts raised concerns, the controversy also served to remind us how hasty trials can result in handling errors. It also underscores the need for Oxford-AstraZeneca’s India partner, Serum Institute of India, to ensure a highly transparent trial for Covishield to dispel any doubt and distrust over its efficacy.
Funding medical research must be top priority: The adenovirus vector platform that has been used in the Oxford vaccine was first developed in the early 1990s and has since then been repeatedly tested and modified in continued research and development. The current vaccine design that could successfully use the existing platform is essentially based on almost three decades of research and testing. With the success of the vaccine in sight, it is important to understand that none of it would have been possible without years of expertise developed by the scientists and the adequate funding and resource support given to medical research.
These researchers are our modern heroes, who have made tremendous sacrifices and given it their all, in order to make a difference to humankind and create history. In all of this, science will be the winner. Let this encourage and inspire our youngsters to be future scientists who can make the impossible possible.
Countries like India, where the shortage of medical research funding has been a perennial problem, can also learn vital lessons from this. Notably, the Department of Health Research in India was given barely three per cent of the total healthcare allocation in the Budget 2020-21. India needs to increase funding and spend more on biomedical research to be able to develop effective drugs and vaccine solutions for multiple diseases in the future.
(The writer is Executive Director of a pharmaceutical firm)







 OpinionExpress.In
OpinionExpress.In












Comments (0)